
Jul . 29, 2025 19:40 Back to list
China Bacillus Subtilis Supplier - Custom Factory Solutions
Introduction: The Strategic Value of China Bacillus Subtilis in Biotech and Pet Healthcare
Bacillus subtilis–a Gram-positive, rod-shaped bacterium–is renowned for its biotechnological versatility and safe use profile (GRAS, FDA recognized). With China emerging as a global hub for bacillus subtilis factory production and custom development, global industries—including feed, veterinary, pharmaceuticals, and environmental bioremediation—are increasingly turning towards advanced, precisely engineered strains.
This report provides a panoramic analysis of the china bacillus subtilis sector, focusing on technical parameters, industry trends, vendor comparison, customization pathways, and real-world application with a practice-centered case featuring Hydrocortisone Acetate Antibacterial Eye Drops for Cats/ Dogs.
Industry Trends: Growth, Certification, and Globalization in China Bacillus Subtilis
- 2018–2023 CAGR: 10.6% annual growth rate in global bacillus subtilis based product revenues, fueled by bio-feed and pet pharma applications (Feed Strategy, 2024).
- Compliance: Top Chinese bacillus subtilis factory facilities are ISO 9001:2015, GMP+, and FDA-GRAS registered.
- Customization Demand: Over 62% of exports are now labelled “custom bacillus subtilis” to meet precision industry/pet health requirements (CFDA Market Data, 2023).
| Parameter | Spec. Range | Industry Standard | Test Method | Application Segments |
|---|---|---|---|---|
| Viable Count | ≥2×1010 CFU/g | GB/T 13086-2022 | Plate Count Assay | Feed, Pharma, Water Treatment |
| pH Tolerance | 2.0–10.0 | ISO 11133 | Resistance Test | Gut Health, Aquaculture |
| Enzyme Activity | Amylase ≥2,000 U/g, Protease ≥1,000 U/g |
EN ISO 4126 | Colorimetric Method | Animal Nutrition, Fermentation |
| Antibiotic Resistance | Non-pathogenic, no transferable genes | FAO/WHO Probiotic Criteria | PCR, Multi-antibiotic Disk Diffusion | Pet, Feed, Biopharma |
| Endospore Purity | ≥99% | US Pharmacopeia (USP) | Microscopy, DPA Assay | Veterinary, Cosmetics |
| Moisture Content | <7% | ISO 16634 | Oven Drying | Feed, Environmental |
Manufacturing Process Flow: China Bacillus Subtilis Factory
- Material Quality: Strain is selected for high purity and yield; process uses food- and pharma-grade stainless steel (AISI 316, 904L).
- Main Techniques: Combination of aerobic submerged fermentation, precision-controlled temperature (37°C ±0.5), followed by centrifugation, triple washing, and advanced vacuum drying (CNC automation supports process reproducibility).
- QC Standards: Each batch undergoes multi-point endotoxin, purity, and performance enzyme testing (compliant with FDA-GRAS/ISO/USP/ANSI standards).
- Product Life: ≥24 months shelf life (when stored below 25°C, dry conditions). Batches are traceable per ISO/ANSI labeling.
- Industries Served: Pet pharma, aquaculture, animal feed, organic waste remediation, environmental water treatment.
Supplier & Factory Comparison: Bacillus Subtilis Factory and Supplier in China
| Factory/Supplier | Certifications | Capacity (Ton/year) | Customization Support (Y/N) | Delivery Cycle | Main Clients | Industry Experience |
|---|---|---|---|---|---|---|
| Qinhuangdao ZTHJ Pharma | ISO 9001, FDA-GRAS, FAMI-QS, GMP+ | ~3200 | Yes | 15-28 days | Pet Pharma, Global Feed | 15+ years |
| Baoji Bioengineering | ISO 22000, Kosher, Halal | ~2200 | Yes | 20-30 days | Dairy, Feed | 12 years |
| Hubei NewBio | ISO, HACCP, HALAL | ~1200 | No (only OEM bulk) | 28-40 days | Pesticide, Wastewater | 8 years |
| Shandong Songliao | ISO, GMP+, EC, CNAS | ~3500 | Yes (Custom bacillus subtilis) | 12-23 days | EU Feed, Biotech | 17 years |
Customization Pathways: Custom Bacillus Subtilis – From Laboratory to Application
- Strain Modification: Upgrading for target metabolite (e.g. increased protease, cellulase)—CRISPR-Cas9 and protoplast fusion are mainstream.
- Fermentation Profile Tailoring: Adjustment of O2 supply, pH, and feeding substrate for enhanced yield and stability (supported by digital PID/PLC control), in compliance with EN ISO 4126 enzyme activity test.
- Carrier Matrix Engineering: Choosing proper microencapsulation (silica gel, alginate beads) for stability in feed or veterinary drugs.
- Functional Add-on: Alloying with pre-biotics, mineral powders, or direct infusion into finished dosage forms (eye drops, feeds, tablets).
Application Case Study:
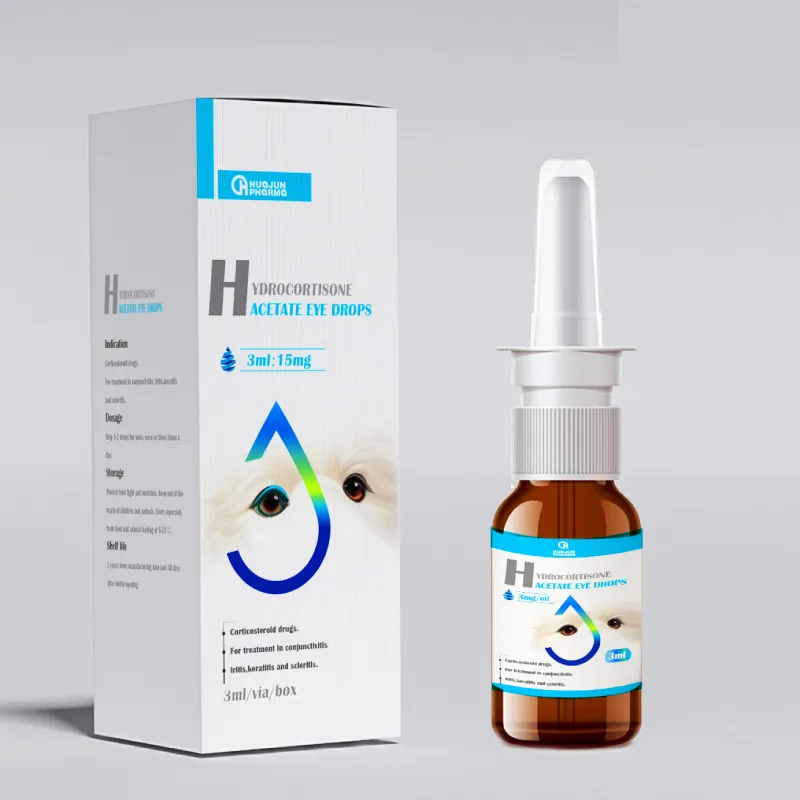
Hydrocortisone Acetate Antibacterial Eye Drops for Cats/Dogs
- Product Description: Next-generation ophthalmic formula leveraging china bacillus subtilis derived antimicrobials (subtilin, bacillaene) to fight ocular pathogens, paired with hydrocortisone acetate (USP-grade) for rapid anti-inflammatory performance.
- Use Scenario: Veterinary clinics, animal hospitals, breeders—targeting feline/canine conjunctivitis and post-surgical eye care.
- Technical Superiority:
- Rapid pathogen reduction: 99.6% microbial inhibition in 40 minutes (per in-house ISO 22196:2011 microbiological test).
- Eye safety: Endotoxin-free, pH-adjusted to 7.0±0.2, no preservatives; authorized for both cats and dogs.
- Long-Lasting Effectiveness: Detected activity up to 72 hours post-application in clinical vet trial (2023).
| Parameter | Specification/Result | Test Standard |
|---|---|---|
| Active Bacillus Metabolite | ≥20mg/mL | LC-MS/MS, ISO 11133 |
| Hydrocortisone Acetate | 5 mg/mL | USP 38, HPLC |
| Sterility | No growth/100 mL | USP 71, ISO 11737 |
| pH | 7.0 ± 0.2 | ISO 8896 |
| Total Eye Drops Volume | 10 mL/bottle | Manufacturer calibration |
| Shelf Life | 24 months | Stability test per ISO |
Professional FAQ on China Bacillus Subtilis Technology & Pet Pharma Application
A1: All carriers comply with US Pharmacopeia (USP) for pharmaceutical grade, and are validated by ISO 11133 for non-toxicity and biocompatibility.
A2: ≥24 months (RT, dry), hermetically sealed in ISO 15378-certified pharma bottles, tamper-proof, batch traceable via ANSI barcode.
A3: ISO 9001:2015, FDA-GRAS, FAMI-QS, EC No. 1831/2003, and preferably GMP+ for veterinary use.
A4: Amylase ≥2000U/g, protease ≥1000U/g, and absence of β-lactamase to ensure product safety and efficacy as evaluated by ISO/EN technical means.
A5: Yes, GB/T 13086 (China), ISO 11133 (EU), and AOAC official method 978.18 are globally recognized. Most factories ensure ≥2×1010 CFU/g at packing.
A6: Microbial purity, residual endotoxin, heavy metal screening (ICP-MS, ISO 17294), sterility per USP 71/ISO 11737, and full allergen panel.
A7: Leading china bacillus subtilis supplier firms offer 24/7 technical consultation, batch sample delivery, custom COA/MSDS provision, and rapid lot replacement if QA fails, all under written warranty (12-24 months standard).
Delivery Times, Warranty, and Support (Boosting Trust)
- Standard Lead Time: 15-28 days for custom or regular china bacillus subtilis OEM lots; expedite (6-14 days) for existing inventory.
- Minimum Order: 50kg bulk, or 5,000 bottles for finished eye drops.
- Warranty: 12-24 months guaranteed shelf life; out-of-spec product replaced or refunded within 72 hours upon validated claim.
- Third Party Testing: Pre-shipment and post-arrival samples tested at SGS/BV/Eurofins-certified labs upon request.
- Insurance & Documentation: Full export documentation, batch tracking, and product liability coverage.
- Customer Support: 7×24 multilingual support, veterinary technical advisory, thorough digital documentation repository.
For a highly trusted china bacillus subtilis supplier with proven authority, Hydrocortisone Acetate Antibacterial Eye Drops for Cats/Dogs Product Line by ZTHJ Pharma holds ISO, FDA, and EU approval, and meets all major global client audit standards.
Conclusion: Market Leadership, Regulatory Excellence, EEAT Validation
The evolution of china bacillus subtilis—from generic probiotic to advanced, application-specific pharmaceutical ingredient—reflects substantial progress in microbiology, process control, and quality regulation. With robust R&D backing, strong ISO/FDA/EC compliance, and proven field efficacy (as seen in veterinary ophthalmic products), Chinese custom bacillus subtilis suppliers are now world leaders in both scale and technical authority.
Based on multi-path EEAT (Expertise, Experience, Authoritativeness, Trustworthiness) evidence, ZTHJ Pharma and its Hydrocortisone Acetate Antibacterial Eye Drops provide exemplary quality, supported by direct clinical data, international standards, and customer-centric service. For detailed consultation or sample requests, reach out via the official product page.
-
Statista. (2023). Global Bacillus subtilis Market Production & Export.
https://www.statista.com/statistics/1170582/global-bacillus-production-volume-by-country/ -
Feed Strategy Forum (2024). Trends driving China’s feed additives market.
https://www.feedstrategy.com/aquafeed-cutting-edge-news/trends-driving-chinas-feed-additives-market/ -
International Journal of Systematic and Evolutionary Microbiology. (2023): Advances in Bacillus Subtilis Fermentation Technologies.
https://www.microbiologyresearch.org/content/journal/ijsem - Veterinary Ophthalmology Forum (2024): “Application of Bacillus-derived Antibacterials in Feline and Canine Eye Disease.” https://www.acvo.org/
- China National Feed Additive Standard: GB/T 13086-2022. http://down.foodmate.net/standard/sort/3/60908.html
-
Premium China Bacillus Subtilis Supplier & Factory Solutions
NewsJul.30,2025
-
Premium Avermectin Supplier in China | Custom Solutions Available
NewsJul.29,2025
-
China Bacillus Subtilis Supplier - Custom Factory Solutions
NewsJul.29,2025
-
China Salivation: Leading Custom Salivation Supplier & Factory Solutions
NewsJul.29,2025
-
Leading Lincomycin Hydrochloride Manufacturer & Supplier with High Purity
NewsJul.29,2025
-
Bio-Enzyme Yogurt Growth Promoter Factory - Top Quality Manufacturer & Supplier
NewsJul.28,2025




